Nickel plating at home with your own hands: technology, because of
Nickel plating, which is a fairly common technological operation, perform for that, to apply a thin layer of nickel on the surface of the metal product. The thickness of such a layer, the value of which can be adjusted, using different techniques, may vary from 0,8 to 55 micron.

Nickel plating is used as a protective and decorative coating, as well as to obtain a sublayer for chrome plating
With the help of nickel plating the metal can form a film, which provides reliable protection against such negative phenomena, as oxidation, development of corrosion processes, reactions, caused by interaction with salt, alkaline and acidic media. In particular, nickel-plated pipes have become very widespread, which are actively used for the production of plumbing products.
Most often subjected to nickel plating:
- metal products, which will be operated outdoors;
- body parts of motorcycles and motor vehicles, including those, for the manufacture of which an aluminum alloy was used;
- equipment and tools, used in general medicine and dentistry;
- metal products, which are operated in water for a long time;
- fencing structures, made of steel or aluminum alloys;
- metal products, exposed to strong chemicals.
There are several used in both manufacturing, and at home methods of nickel plating of metal products. The methods of nickel plating of metal parts are of the greatest interest in practical terms, which do not require the use of complex technological equipment and are sold at home. Such methods include electrolytic and chemical nickel plating.
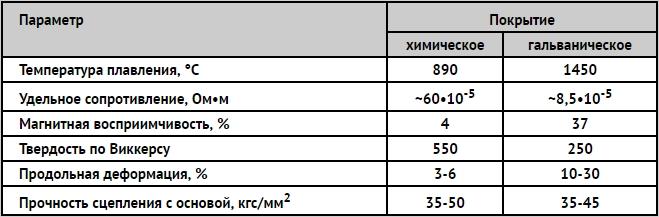
Properties of galvanic and chemical coating of nickel
Electrolytic nickel plating
The essence of the technology of electrolytic nickel plating of metal parts, has another name - "galvanic nickel plating", can be considered as an example, how is the copper plating of the surface of the metal product. This procedure can be performed using an electrolytic solution, and without him.
Detail, which will be further processed in electrolytic solution, is subjected to careful processing, for which the oxide film is removed from its surface with the help of sandpaper. Then the treated product is washed in warm water and treated with soda solution, then washed again with water.

Large parts are best cleaned with a sandblasting machine
The nickel plating process itself is performed in a glass container, in which the aqueous solution is poured (electrolyte). The composition of such a solution contains 20% copper sulphate and 2% sulfuric acid. Workpiece, on the surface of which it is necessary to apply a thin layer of copper in the electrolyte solution is placed between two anodes of copper. To start the copper plating process, an electric current must be applied to the copper anodes and the workpiece, the value of which is calculated, based on the indicator 10-15 mA per square centimeter of detail area. A thin layer of copper on the surface of the product appears after half an hour of its presence in the electrolyte solution, and such a layer will be the thicker, the longer the process.
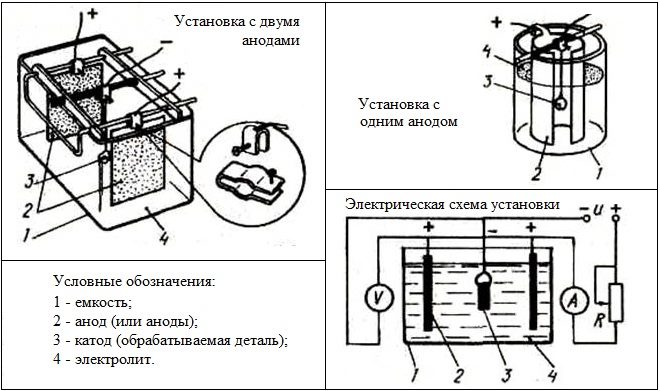
Installation diagram for electrolytic nickel plating
It is possible to put a copper layer on a product surface and on other technology. To do this, make a brush of copper (you can use stranded wire, having previously removed the insulating layer from it). Such a brush, made with their own hands, should be fixed on a wooden stick, which will serve as a handle.
Product, the surface of which is pre-cleaned and degreased, placed in a container made of dielectric material and filled with electrolyte, as which you can use a saturated aqueous solution of copper sulfate. The self-made brush is connected to the positive contact of the electric source, and the processed detail - to its minus. After that, start the copper plating procedure. It is, that brush, which is pre-soaked in electrolyte, spend over the surface of the product, without touching her. Apply coating, using this technique, can be in several layers, which will form a layer of copper on the surface of the product, on which there are almost no pores.
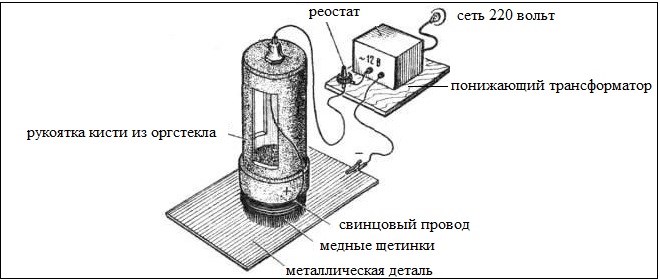
Scheme of a simple device for coating
Electrolytic nickel plating is performed by a similar technology: in its implementation also uses an electrolyte solution. The same, as in the case of copper plating, the workpiece is placed between two anodes, only in this case they are made of nickel. Anodes, placed in a solution for nickel plating, are connected to the positive contact of the current source, and the product, suspended between them on a metal wire, - to minus.
For nickel plating, including performed with their own hands, electrolytic solutions of two main types are used:
- water solution, which includes nickel sulfate, sodium and magnesium (14:5:3), 2% boric acid, 0,5% table salt;
- solution based on neutral water, containing in its composition 30% nickel sulfate, 4% nickel chloride, 3% boric acid.
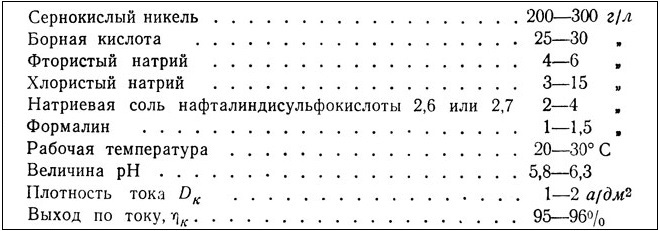
Brilliant nickel electrolyte with the addition of organic brighteners (sodium salts)
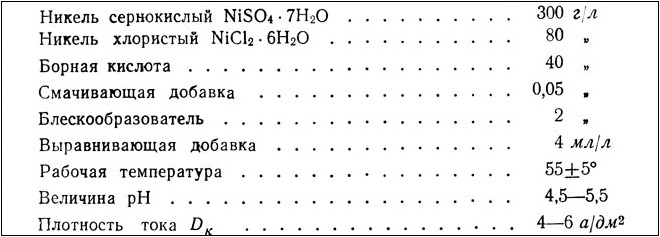
Brilliant nickel leveling electrolyte. Suitable for surfaces with low cleaning class
To prepare an electrolytic solution, the dry mixture of the above elements is poured into one liter of neutral water and mixed thoroughly. If a precipitate formed in the resulting solution, get rid of it. Only then can the solution be used to perform nickel plating.
Processing with this technology usually takes half an hour, at the same time use a current source with a voltage of 5.8-6 V.. The result is a surface, covered with uneven matte gray color. To make her beautiful and brilliant, it is necessary to clean and perform its polishing. It should be borne in mind, that such technology cannot be used for details, characterized by high surface roughness or having narrow and deep holes. In such cases, the coating of the surface of the metal product with a layer of Nickel should be performed by chemical technology, which is also called blackening.
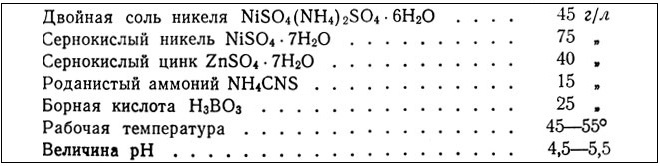
Electrolyte for the deposition of black nickel
The essence of the technological operation of blackening is, that the surface of the product is first applied an intermediate coating, which can be based on zinc or nickel, and on the upper part of such a coating is formed a layer of black Nickel with a thickness of not more 2 micron. Nickel plating, made by blackening technology, looks very beautiful and provides reliable protection of the metal from the negative effects of various environmental factors.
In some cases, the metal product is subjected to two technological operations at the same time, such as nickel plating and chrome plating.
Chemical nickel plating
The procedure of chemical nickel plating of metal products is performed according to the following scheme: the workpiece is immersed in boiling solution for some time, as a result, nickel particles settle on its surface. When using this technology electrochemical effect on the metal, of which the part is made, is absent.
The result of using such nickel plating technology is the formation on the surface of the workpiece of the nickel layer, which is strongly bound to the parent metal. The greatest efficiency of this method of nickel plating can be achieved in those cases, when it is used to process objects, made of steel alloys.
Kit for nickel coating by chemical means
It is not difficult to perform such nickel plating at home or even in a garage. The nickel plating procedure takes place in several stages.
- Dry reagents, from which the electrolytic solution will be made, mixed with water in an enamel dish.
- The resulting solution is brought to a boil, and then sodium hypophosphite is added to it.
- Product, which must be processed, placed in electrolytic solution, and do so, so that it does not touch the side walls and bottom of the tank. In fact, it is necessary to make a household appliance for nickel plating, the design of which will consist of an enameled container of appropriate volume, as well as a dielectric bracket, on which the processed detail will be fixed.
- The duration of boiling of the electrolytic solution, depending on its chemical composition can be from one to three hours.
- After completion of the technological operation, the already nickel-plated part is removed from the solution. Then it is washed in water, which contains slaked lime. After thorough washing, the surface of the product is polished.

The process of nickel plating at home
Electrolytic solutions for nickel plating, to which it is possible to expose not only steel, but also brass, aluminum and other metals, Be sure to include the following elements in your chemical composition: Nickel chloride or sulfate, sodium hypophosphite of various acidity, any of the acids.
To increase the nickel plating rate of metal products, composition to perform this technological operation add lead. Usually, in one liter of electrolytic solution perform a nickel coating of the surface, the area of which is 20 sm2. In electrolytic solutions with higher acidity, nickel-plating of ferrous metal products is carried out, and in alkaline process brass, carry out nickel plating of details from aluminum or stainless steel.
Some nuances of technology
Performing nickel plating of brass, steel products of various brands and other metals, some nuances of this technological operation should be taken into account.
- Nickel film will be more stable, if it is applied to a pre-copper plated surface. The nickel-plated surface will be even more stable in that case, if the finished product will be subjected to heat treatment, which consists in its endurance at temperature, exceeding 450 °.
- If hardened steel parts are nickel-plated, then you can heat and keep them at room temperature, not exceeding 250-300 °, otherwise they may lose their hardness.
- When nickel plating products, differing in large size, there is a need for constant stirring and regular filtration of the electrolytic solution. This complexity is especially characteristic of nickel plating processes, are not performed in industrial, and at home.
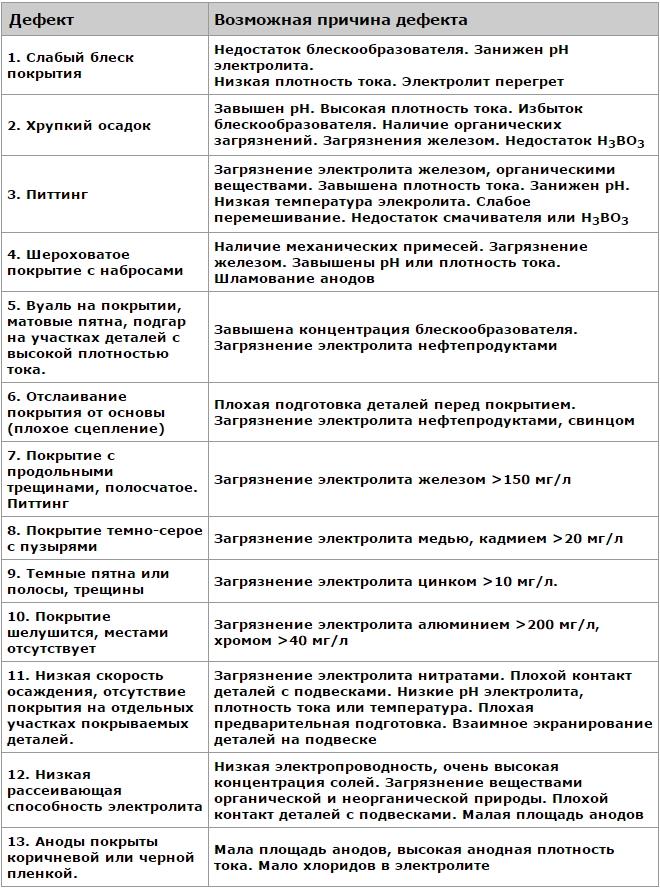
Causes of nickel plating defects
By similar technology with nickel plating, you can cover the brass, steel and other metals with a layer of silver. Coatings of this metal are applied, in particular, for fishing tackle and other products, to prevent them from fading.
The procedure for applying a layer of silver on steel, Brass and other metals differ from traditional nickel plating not only in temperature and holding time, but also that, that it uses an electrolytic solution of a certain composition. This operation is performed in solution, whose temperature is 90 °.

Nickel-plated brass fittings
To prepare the solution with your own hands, by means of which on steel, brass and other metals are applied a layer of silver, just take a few simple steps.
- In 10% aqueous salt solution add pharmacy lapis.
- Silver precipitate, which fell out in solution, washed, mixed with 2% hyposulfite and filtered.
- The resulting mixture is mixed with chalk dust and brought to a creamy state.
Such a mixture, which can be stored for only a few days, the surface of a metal product is rubbed, until a thin layer of silver is formed on it.

The resulting coating is easily polished to a shine
You can prepare a powder for silvering, which will not lose its characteristics for six months. To obtain such a powder must be mixed 15 grams of lapis, 55 grams of citric acid and 30 grams of ammonium chloride. All components after mixing should be ground to dust. The resulting powder is stored in dry form.
Nickel plating of such metal is quite difficult, like aluminum. Components, which are a part of electrolytic solution for nickel plating of products from this metal, expensive, but even their use does not guarantee that, the nickel layer formed on the product will not go bubbles. Brilliant nickel plating, if it is exposed to aluminum, may break the finished coating, therefore in house conditions such processing is carried out in the conditions of weak adhesion.