The concept of resistivity of substances, table of resistance of metals and properties of copper
What is the resistivity of a substance? To answer this question in simple words, it is necessary to remember the course of physics and to present the physical embodiment of this definition. An electric current is passed through the substance, and it, in turn, prevents with some force the passage of current.
The concept of resistivity
It is this value, which shows how much the substance interferes with the current and there is a resistivity (latin letter "rv"). In the international system of units, resistance is expressed in Omaha, multiplied by a meter. The formula for the calculation sounds like this: "Resistance is multiplied by the cross-sectional area and divided by the length of the conductor".
The question arises: "Why another resistance is used when finding the resistivity?». The answer is simple, there are two different values - resistivity and resistance. The second shows how much the substance is able to prevent the passage of current through it, and the first shows almost the same, only it is no longer a matter in the general sense, and about the conductor with a specific length and cross-sectional area, which are made of this substance.
Inverse value, which characterizes the ability of matter to transmit electricity is called the specific electrical conductivity and formula, according to which the resistivity is calculated is directly related to the conductivity.
The use of copper
The concept of resistivity is widely used in calculating the conductivity of electric current by various metals. Based on these calculations, decisions are made on the feasibility of using a metal for the manufacture of electrical conductors, used in construction, instrumentation and other industries.
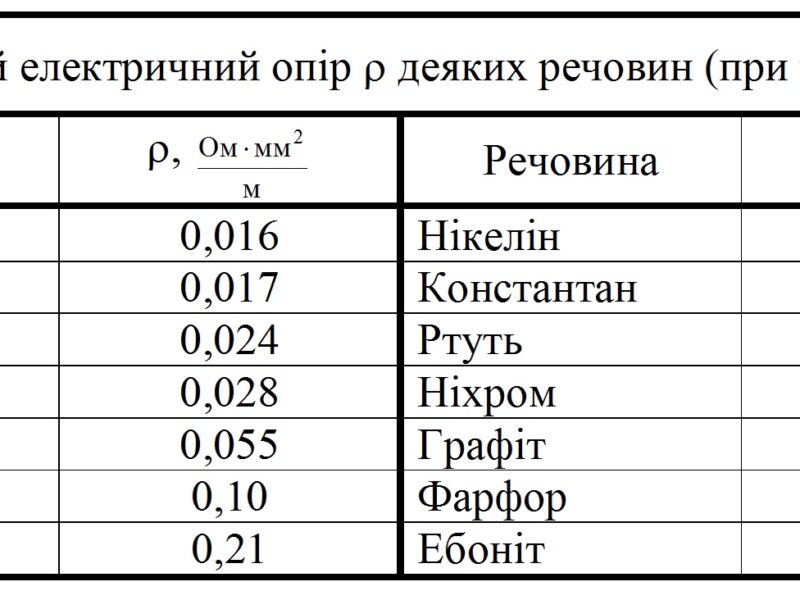
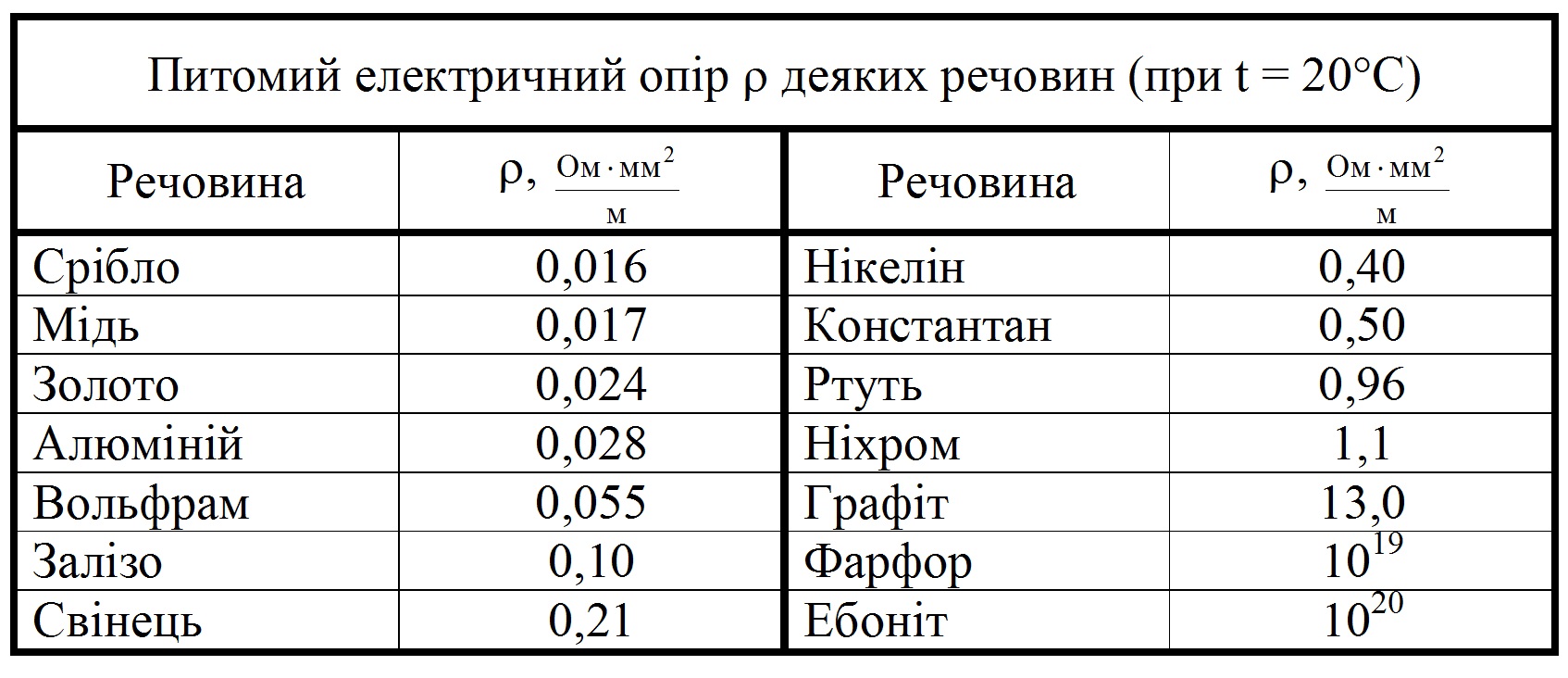
Metal resistance table
Існують певні таблиці в яких зведені воєдино наявні відомості про пропущенні і опір металів, usually, these tables are designed for certain conditions.
In particular, widely known table of resistance of metal single crystals at a temperature of twenty degrees Celsius, as well as a table of resistance of metals and alloys.
These tables are used to calculate various data in the so-called ideal conditions, to calculate values for specific purposes you need to use formulas.
Copper. Its characteristics and properties
Description and properties of the substance
Copper is a metal, which has long been discovered by mankind and has also long been used for various technical purposes. Copper is a very malleable and malleable metal with high electrical conductivity, this makes it very popular for making various wires and conductors.
Physical properties of copper:
- melting point - 1084 degrees Celsius;
- boiling point - 2560 degrees Celsius;
- density at 20 degrees - 8890 kilogram divided by cubic meter;
- specific heat at constant pressure and temperature 20 degrees - 385 kJ / J * kg
- electrical resistivity - 0,01724;
Brands of copper
This metal can be divided into several groups or brands, each of which has its own properties and its application in industry:
- Brands M00, М0, M1 - great for the production of cables and conductors, during its remelting oxygen supersaturation is excluded.
- Brands M2 and M3 are cheap options, which are intended for small hire and satisfy the majority of technical and industrial tasks of small scale.
- Brands M1, М1ф, М1р, М2р, M3r are expensive copper brands, which are made for the concrete consumer with specific requirements and inquiries.
Brands differ in several respects:
type of supply;
- oxygen saturation;
- the difference in resistance;
- the presence of impurities;
- degree of thermal conductivity;
Influence of impurities on the properties of copper
Impurities can affect mechanical, technical and operational properties of products.
- Mechanical properties. Such substances, like iron, bismuth, lead or oxygen, affect the ductility of copper. Some sparingly soluble impurities affect the preservation of the structure of the substance with increasing temperature. Example, lead or bismuth makes copper very brittle, but adding at least a small amount of silver (five hundredths of a percent) significantly increases the fusibility of copper, that is, even at high temperatures, its crystal lattice remains unchanged, at the same time there is no rubbing of heat - or electrical conductivity.
- Technical properties. These include pressure treatment at different temperatures and fusibility (welding) substances. In the presence of sparingly soluble copper impurities, zones of special fragility appear at high temperatures, this makes pressure treatment very difficult, but, in brands M1 and M2 the required plasticity is achieved due to the low content of impurities. If we talk about pressure at low temperatures, then this technology is used in the production of wire rod (wire) and for different brands the ability to extract is also different.
Performance properties. Under standard operating conditions, different brands behave in exactly the same way, but due to the content of hydrogen and oxygen in different brands, the conditions apply when the temperature rises. In particular, oxygen begins to adversely affect copper with increasing ambient temperature, and hydrogen when the substance itself is heated to two hundred degrees.
In conclusion, it should be emphasized, that copper is a unique metal with unique properties. It is used in the automotive industry, manufacture of elements for the electrical industry, electrical appliances, consumer goods, hours, computers and more. With its low resistivity, this metal is an excellent material for the manufacture of conductors and other electrical appliances.. With this property, copper surpasses only silver, but due to the high cost, it has not found the same application in the electrical industry.