Gilding at home: methods of applying gilding
Learning to perform gilding at home, which is not so difficult, as it may seem at first glance, you will be able to bring a second life to your favorite jewelry made of copper and silver. Ware, made of gold, have been very popular with women for many years, and in men. To have such products without significant costs for their purchase, enough to master the technology of gilding.

It is possible to cover with gilding as non-ferrous metals, and steel or cast iron
On products from which metals it is possible to put a gilding layer
The most common process is the gilding of silver, but gold plating can be applied to the surface of other metals. So, gilding can be applied to copper products, brass and zinc, as well as iron and steel, etc..
When asked about that, how to gild metal at home, there is no clear answer. It all depends, products from which metal must be subjected to such processing. The choice of gilding technology, carried out at home, the result is also affected, which must be achieved.

With the help of gilding, you can give ordinary things a completely different look
Various methods can be used for gilding metal, the most common of which are:
- rubbing the surface of the product with a solution of gold chloride;
- gilding, performed by immersing the product in a solution with zinc contact;
- galvanic gilding.
Each of these methods of gilding, performed at home, requires the use of certain chemical reagents, tools and equipment.
Preparation and use of chlorinated gold
To cover a layer of gilded metal, often use a solution, called gold chloride. To prepare this solution, gold is dissolved in "royal vodka", which is a mixture of hydrochloric and nitric acids. Hydrochloric and nitric acids are taken in proportions 3: 1. Gold is placed in this composition, and then evaporate the liquid. Perform the procedure of evaporation of liquid from this solution should be very careful, to avoid burns to the skin and respiratory tract. Dry matter, remaining after evaporation, just is chlorinated gold.

When evaporating, separate the open flame from the solution tank, example, making a layer of asbestos crumbs, poured into a separate vessel
Before applying chlorinated gold for gilding, it must be mixed with a solution of potassium cyanide and whipped with chalk, resulting in a paste-like mass. Such a gruel, using a brush, cover the product, after which it is kept for some time, and then thoroughly washed and polished.
To gild steel, chlorinated gold is mixed with ether. Covered with this composition, the product is left for some time, until the ether completely evaporates, and then the treated surface is simply rubbed with a cloth to give a golden sheen.
Using chlorinated gold, pre-mixed with ether, various inscriptions and patterns can be applied to a metal object. In order to carry out such a procedure, a goose feather is immersed in the resulting solution and the necessary inscriptions and patterns are performed, which after evaporation of ether and polishing will sparkle with golden luster.

Large surfaces are gilded with a soft brush
As mentioned above, gold plating is often applied to silver, for which chlorinated gold can also be used. To perform chemical gilding of products made of this metal, it is necessary to prepare the mixture, which includes the following components:
- chlorne gold - 10 grams;
- potassium cyanide - 30 grams;
- table salt - 20 grams;
- soda - 20 grams;
- water - 1,5 l.
Chemical gilding, to which it is necessary to expose silver, can also be performed using a mixture of:
- gold chloride - 7 grams;
- potassium ferrocyanide - 30 grams;
- potassium carbonate - 30 grams;
- table salt - 30 grams;
- water - 1 l.
The procedure of spraying a layer of gold on the metal surface using chemical solutions is performed in the following sequence.
- The workpiece is pre-calcined.
- The surface of the object is first treated with a solution of sulfuric acid, and then nitric acid.
- The pickled product is immersed in the mixture for a moment, consisting of sulfur, nitric and hydrochloric acids.
- After treatment in a mixture of acids, the product is rinsed with water, then immersed in mercury and finally in water, where it is kept 30 seconds.
- After the container with water, the product is placed in a solution for gilding, withstand the required time, then washed with water and dried in sawdust.
Application of zinc contact
To get a thicker gilded layer, use zinc contact. This method is possible, example, cover with a layer of gold silver. A composition of such components is prepared for gilding, as:
- chlorne gold - 15 grams;
- carbon salt - 65 grams;
- yellow blood salt - 65 grams;
- table salt - 65 grams;
- water - 2 l.

It will take some time to dissolve all the components of the composition
Ware, made of copper and brass, covered with gold in a solution of the following composition:
- chlorne gold - 2 grams;
- potassium hydroxide - 6 grams;
- potassium cyanide - 32 grams;
- phosphorus-sodium salt - 10 grams;
- water - 2 l.
Items, on the surface of which it is necessary to apply a layer of gilding, thoroughly cleaned of dirt and grease, then they are placed in a preheated composition for gilding. The products already there are connected with a zinc rod, which acts as a contact.
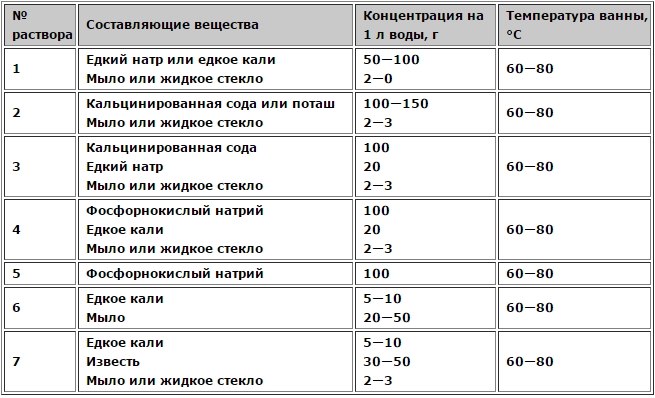
Compositions of degreasing solutions
To gild, applied to the surface of steel products, zinc and tin, was high quality and had good adhesion, before gilding they must be subjected to copper plating.
Galvanic method of gilding
The strongest and qualitative layer of gilding allows to receive a galvanic covering with gold, which performs in special electrolytic solutions. This gilding technology is very similar to galvanizing, as electroplating and similar electrochemical processes are used for its realization.
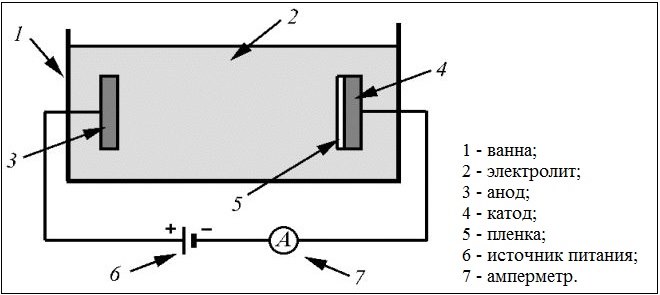
Scheme of galvanic bath
Depending on the chemical composition of the solution, in which galvanization is performed, the formed gilding can have a reddish or light yellow shade. Basically gilding of metal products by this technology is performed in solutions of two types.
Electrolytes for gilding of the first type are prepared in the following sequence.
- IN 700 milliliters of water is dissolved 60 grams of sodium phosphate.
- IN 150 milliliters of water is diluted 2,5 grams of gold chloride.
- In others 150 milliliters of water is dissolved 1 grams of potassium cyanide and 10 grams of sodium sulfate.
- First, carefully mix the first two solutions, and then add a third to the resulting mixture.
To gild silver or any other metal by this method, the prepared composition is brought to temperature 50-62 ° and use a platinum anode for the process. After depletion of such electrolyte for gilding, chlorinated gold is added to it.
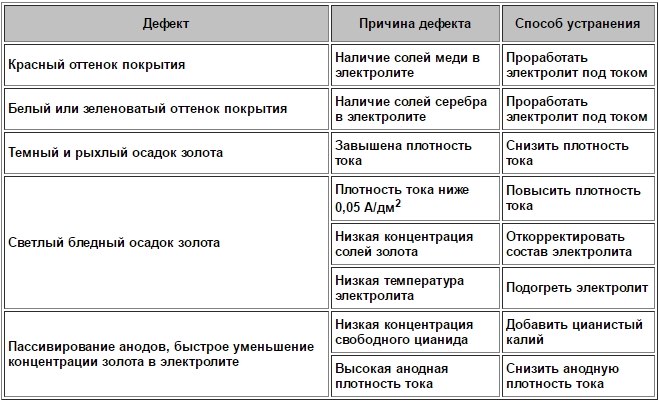
Defects in the use of gilding electrolytes and methods of their elimination
The second type of electrolyte for gilding is called "golden bath Zelm". Silver is gilded in this solution, steel, tin products, copper, brass, chrysanthemum metal. Preparation of this electrolyte for gilding occurs in several stages.
- Bring to a boil in a porcelain container 30 milliliters of water, mixed with crystalline sodium carbonate and ferrous potassium (taken by 1 gram).
- Ammonia-coated explosive gold is added to the resulting solution and boiled for twelve minutes..
- After the formation of a red fluffy precipitate, the resulting liquid, which should have a rich golden color, filtered.
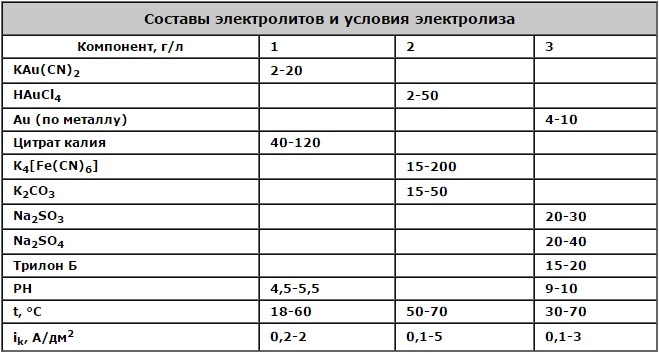
Less toxic are conventionally becyanide gilding electrolytes, the characteristics of which are given in the table
Treatment in such an electrolyte for gilding is carried out for 15-16 hours, using the Daniel element and weak currents. As a result, an impressive matte gilding is formed on the surface of the metal product.
Concerned about it, what is gilding and how to do it, should be considered, that various methods are used for its implementation, only some of which are described above. Choosing the optimal from such technologies, a number of factors must be taken into account, as well as focus on the desired result.